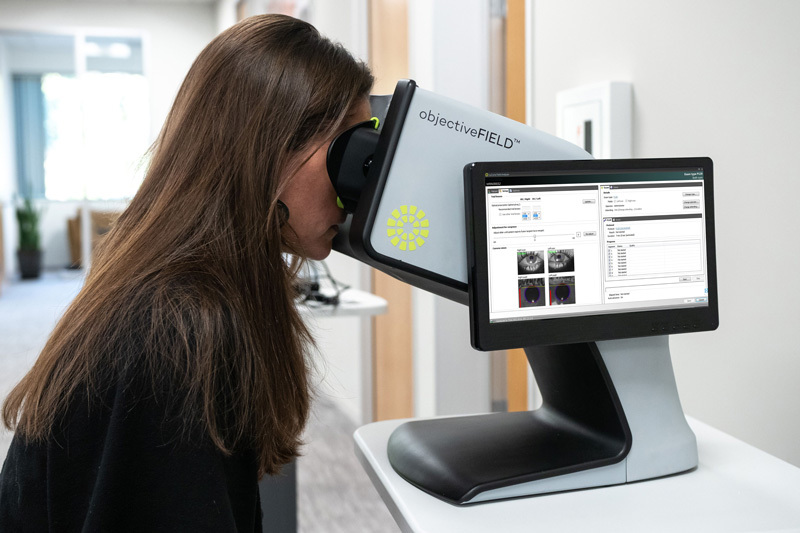
objectiveFIELD®
Finally! Patients no longer have to press a button.
objectiveFIELD® is the first and only truly objective perimeter FDA 510(k) cleared to assess visual field abnormalities.

Overview
The objectiveFIELD® Analyzer (OFA®) utilizes a novel method called multifocal pupillographic objective perimetry (mfPOP), which is analogous to multifocal VEP (mfVEP), but without electrodes.
The objective, bilateral exam is well tolerated by patients and exam times are predictable, with test-protocols as fast as 90 seconds, for both eyes. Normal blinking during the exam is also well tolerated.
Both eyes are tested individually but concurrently (a dichoptic presentation).
- Objective: No button to push, no cognitive / motor response required
- Bilateral: Both eyes tested at the same time, no eye patch
- Novel stimuli: Less susceptible to refractive error, gaze error and lens brunescence from cataracts
- Easy to administer: Monitor patient compliance in real-time
Clinical Applications
objectiveFIELD® is an FDA (510k) Cleared device for the assessment of visual field abnormalities
objectiveFIELD is not FDA cleared for the specific diagnosis of any condition.
Clinical Benefits
Completely objective, no button to press
Bilateral exam, no eye patch needed
Good correlation of glaucomatous RNFL loss with visual field loss
Regulatory
FDA 510(k) Cleared | CPT Code 92083
Health Canada Licensed
Pharmaceuticals and Medical Devices Agency (Japan)
CE Mark pending
What Visual Field Test Protocols Are Available?
OFA includes test protocols for 30° & 24° (together), 15° and 10°. The OFA 30 test protocol delineates a 24° field at the same time, in the same report, as no extra time is required to test a 30° visual field vs 24°.
What Does objectiveFIELD® Measure?
Using a combination of innovative hardware and software, each eye’s field of vision is optically separated, and the patient’s view is fused (cyclopean view).
Novel stimuli are presented to each eye individually, interleaved, with non-overlapping timing, every ¼ second.
Cortically mediated pupillary responses are recorded by video cameras under infra-red light and analyzed using sophisticated pupil tracking (not gaze tracking).
Sensitivity is measured objectively from the amplitude of the pupillary response (relative change in pupil size) and converted to the familiar decibel scale.
Delay (time-to-peak constriction of the pupillary responses) is also measured objectively, in milliseconds, which is new information not available in any other perimeter.
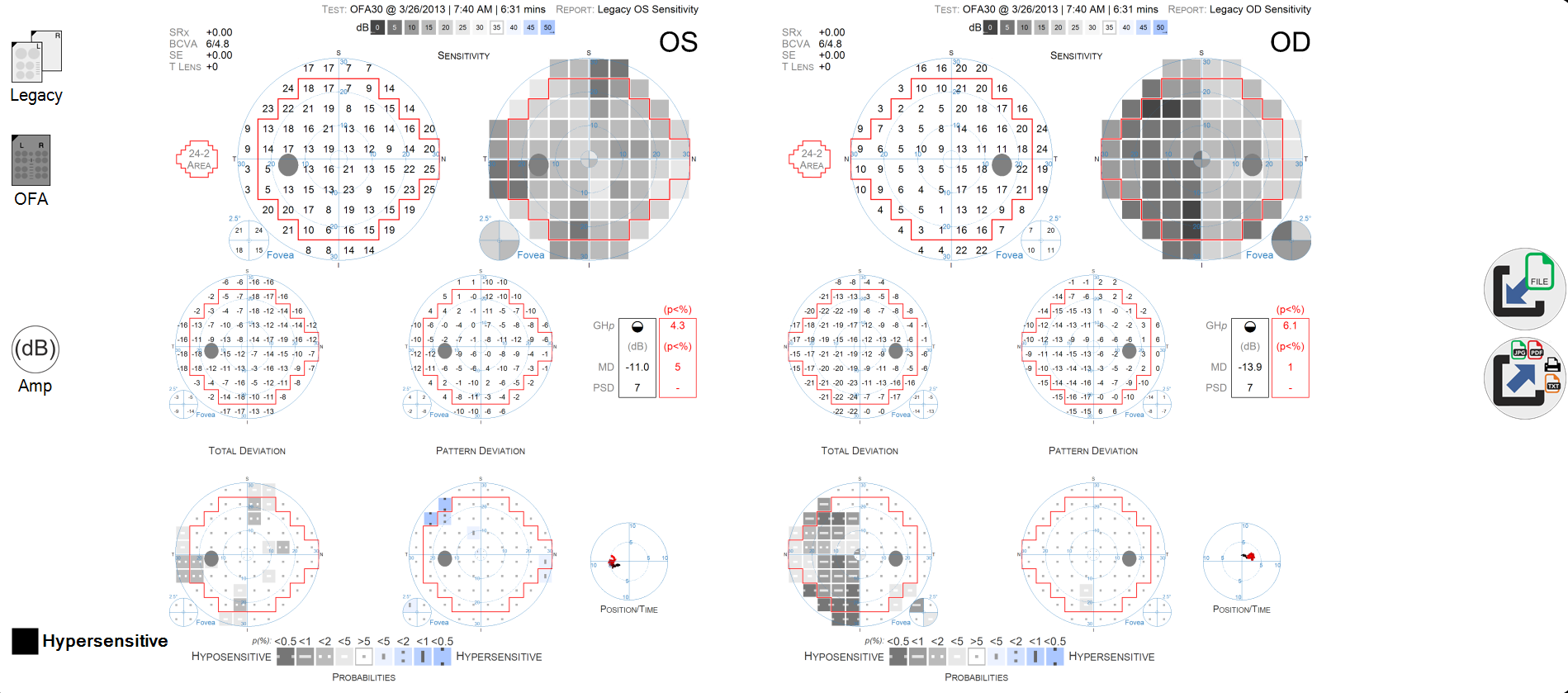
The result is four visual fields per exam.
- OD sensitivity
- OS sensitivity
- OD delay
- OS delay
Reporting
OFA reports are presented in a classic format for both sensitivity and delay, with familiar grey-scale and numeric maps, as well as a table of global VF indices including MD, PSD, SF, CPSD etc.
Ted Maddess, PhD, discusses the objectiveFIELD Analyzer at AAO Chicago
Scientific and Clinical Publications
How does objectiveFIELD work?
How long does a test take?
Two High-Resolution test-protocols (30° & 15°) take approximately 9-10 mins, each, for both eyes, concurrently. These tests are administered as 9 shorts steps (~40 seconds each / ~6:30 actual test time). The patient can take a short break between steps while the system checks the reliability of the data collected in each step. Cadence of the exam is controlled by the operator.
High-Efficiency test-protocols (30° & 10°) take only 90 seconds, also for both eyes, administered as one continuous 90 second step.
How does objectiveFIELD compare to standard automated perimetry?
Responses are measured objectively using a patented “Clustered Volley”, multifocal pupillographic objective perimetry method (mfPOP). OFA® stimuli covers more than 99% of the 30-2 retinal area, while SAP Goldman III stimuli only samples approximately 0.5% of the 30-2 area with the assumption that the remaining 99.5% of the non-sampled central area is well represented by the small stimuli.
Are there any disposables used?
Can the device accommodate smaller faces?
Is refractive correction required?
Product Specifications
| Fundamental Method | Multifocal pupillographic objective perimetry (mfPOP) |
| Intended Clinical Purpose and Use | Visual field examination to measure visual field abnormalities |
| Visual System Stimulus | Patented clustered volley, sparse, multifocal stimulus |
| Visual Function Assessment | Regression-based multifocal analysis |
| Visual Field Defect Assessment | Population sample normal database comparison |
| Test Options | 30°, 24°, 15°, 10° tests |
| Trial Lenses | +3D, -3D, +6D, -6D, +9D, -9D |
| Background Brightness | 10 cd/m2 |
| Regulatory |
FDA 510(k) Cleared K063310 | Health Canada Licensed Pharmaceuticals and Medical Devices Agency (Japan) CE Mark pending |
Intended Purpose of Device & Indications for Use
This device is not intended to diagnose any disease. Use of the device in such a manner may result in misdiagnosis of serious pathologies. It is solely the physician’s or operator’s responsibility to interpret the test results.
The intended use of the device is to aid in the measurement of visual field abnormalities.
The device is intended for use by healthcare professionals or paraprofessionals in either a professional healthcare environment or other places where patients are present.
objectiveFIELD® should not be used to examine the following category of patients, this category may include, but not be limited to: patients with irregular shaped and stationary pupils; patients with Horner’s syndrome or other disorders affecting pupil response; patients with unusually small pupils or patients treated by drugs that would affect the pupil reactivity.



